Abstract
Introduction
Ibrutinib, a first-in-class, oral, covalent inhibitor of Bruton's tyrosine kinase, is approved in several countries as monotherapy for adults with WM, based on the high response rate (90.5%) observed in a phase 2, single-arm trial of symptomatic patients who had received ≥ 1 prior therapy (NCT01614821; Treon SP, et al. N Engl J Med. 2015;372:1430-1440). In the phase 3 iNNOVATE trial (NCT02165397; Dimopoulos MA, et al. N Engl J Med. 2018;378:2399-2410), adding rituximab to ibrutinib (IR) led to a statistically significant improvement in progression-free survival (PFS) compared with placebo-rituximab, in both treatment-naïve (TN) patients and those with disease recurrence. This analysis examined the relative treatment effect of IR versus other RW treatment regimens used in daily clinical practice in TN and relapsed/refractory (R/R) WM. An adjusted comparison was conducted using patient-level data from the iNNOVATE trial and the Lyon-Sud RW database in France.
Methods
The Lyon-Sud database holds medical records for patients with WM diagnosed between 1980 and 2017 from the Centre Hospitalier Lyon-Sud. PFS and overall survival (OS) outcomes were compared between the IR arm of iNNOVATE and RW physicians' choice (PC) treatment in Lyon-Sud (excluding RW ibrutinib). Kaplan-Meier survival curves by patient cohort were generated for both end points. A multivariate Cox proportional hazards model was fitted on the pooled patient-level data from both sources to estimate adjusted hazard ratios (HRs) for effect of IR on PFS and OS versus RW treatment, with age, sex, and treatment line as covariates. The unit of observation for the RW databases was the treatment line (rather than the patient) number, whereby RW patients receiving multiple treatment lines contributed to multiple observations, and baseline was defined as the line-specific treatment start date.
Results
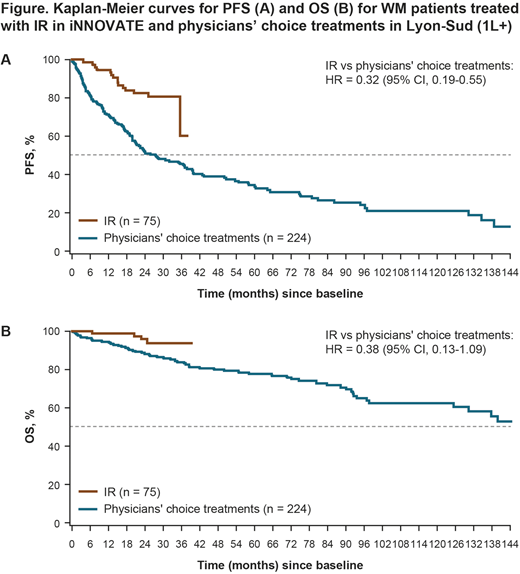
Overall, 242 treatment lines from patients with WM were identified from the Lyon-Sud database; 224 were analyzed. Baseline characteristics were comparable between the IR arm of iNNOVATE (n = 75) and the RW cohort: 62.7% versus 66.5% ≥ 65 years of age; line of therapy, 45.3% versus 48.7% TN [1L], 25.3% versus 25.0% second-line [2L], and 29.3% versus 26.3% third-line or later [3L+]. Median follow-up was 26.7 months for iNNOVATE and 68.5 months for the RW cohort.
Non-ibrutinib PC regimens in the RW cohort included rituximab (n = 51 treatment lines), chemotherapy (n = 66; including chlorambucil, n = 31), rituximab-cyclophosphamide-dexamethasone (n = 35), rituximab-CHOP/CHOP-like (n = 21), rituximab-chlorambucil (n = 15), fludarabine-cyclophosphamide-rituximab (n = 14), bendamustine-rituximab (n = 10), other rituximab-containing chemoimmunotherapy (n = 10), and rituximab-targeted agent (n = 2). Two ibrutinib lines were excluded from the RW cohort analysis.
Figures A and B show the observed 1L+ Kaplan-Meier curves for IR versus RW PC therapy for all analyzed patients with WM (unadjusted HRs: 0.32 [95% confidence interval (CI), 0.19-0.55] for PFS and 0.38 [95% CI, 0.13-1.09] for OS). After adjusting for differences in patient characteristics, HRs (1L+ therapy) became 0.28 (95% CI, 0.16-0.48; p < 0.001) for PFS and 0.29 (95% CI, 0.09-0.93; p = 0.037) for OS. Restricting the analysis to 1L treatment only (Lyon-Sud n = 109; iNNOVATE n = 34), the adjusted HRs were 0.25 (95% CI, 0.09-0.70; p < 0.009) for PFS and 0.20 (95% CI, 0.02-2.00; p = 0.170) for OS; respective unadjusted HRs were 0.31 (95% CI, 0.12-0.78) and 0.30 (95% CI, 0.04-2.34). In the 2L+ setting (Lyon-Sud n = 115; iNNOVATE n = 41), the adjusted HRs were 0.28 (95% CI, 0.15-0.56; p < 0.001) for PFS and 0.34 (95% CI, 0.08-1.35; p = 0.126) for OS; respective unadjusted HRs were 0.31 (95% CI, 0.16-0.61) and 0.39 (95% CI, 0.12-1.31).
Conclusions
In the absence of randomized controlled trial data for ibrutinib versus other treatment regimens for WM, these adjusted comparisons of clinical trial and RW patient-level data suggest that IR significantly improves both PFS and OS versus RW PC regimens as 1L+ therapy. These results help inform physicians on the standard of care for WM in clinical practice.
Funding Source:
This project was sponsored by Janssen Pharmaceutica NV, and Pharmacyclics LLC, an AbbVie Company. The real-world databases are independently owned. Writing assistance was provided by Emma Fulkes of PAREXEL and funded by Janssen Pharmaceutica NV.
Karlin:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support. Besson:Janssen Pharmaceutica NV: Employment. Tapprich:Janssen Pharmaceutica NV: Employment. Garside:Janssen Pharmaceutica NV: Employment. Salles:Roche: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; AbbVie: Honoraria; Acerta: Honoraria; Amgen: Honoraria; Novartis: Consultancy, Honoraria; Merck: Honoraria; Pfizer: Honoraria; Takeda: Honoraria; Servier: Honoraria; Janssen: Honoraria; Gilead: Honoraria; Epizyme: Honoraria; Morphosys: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal